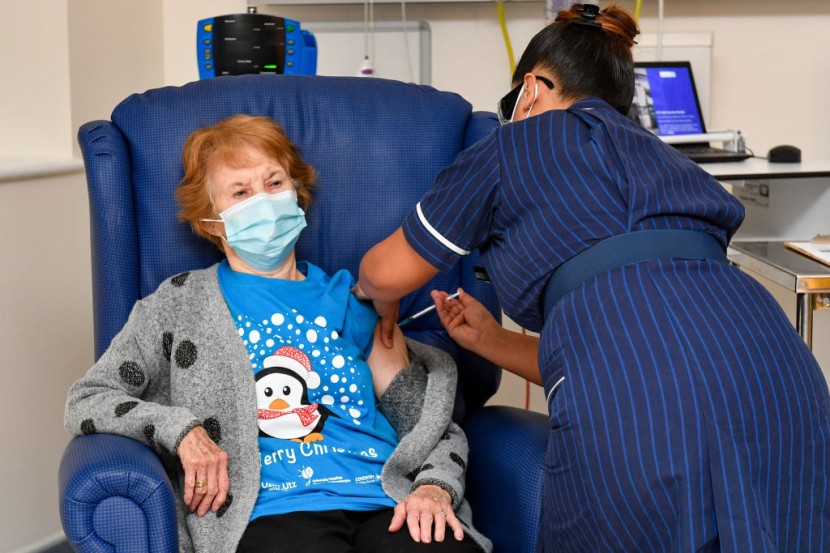
United Kingdom regulators say people who have a notable history of allergic reactions should not be administered the new Pfizer-BioNTech COVID-19 vaccine. At the same time, they probe into two adverse reactions on the first day of the UK's mass immunization program.
People With Allergic Reactions Should Not Take the Vaccine
Britain's medical director for the National Health Service (NHS) Stephen Powis stated health officials were acting on the Medical and Healthcare Products Regulatory Agency's advisory, the country's medicines regulator.
According to Powis in a statement, "As is common with new vaccines, the MHRA has advised, on a precautionary basis, that people with a significant history of allergic reactions do not receive this vaccination after two people with a history of significant allergic reactions responded adversely yesterday. Both are recovering well," reported ABC News.
The two allergic reactions informed by the UK health care workers inoculated against COVID-19 will be evaluated by the Food and Drug Administration (FDA) as it deliberates whether to authorize Pfizer's vaccine in the United States. The incident should not be surprising, according to medical experts.
The UK's Medicines and Healthcare Products Regulatory Agency updated its advisory on Wednesday to recommend individuals who have a history of significant allergic reactions to forgo the novel coronavirus vaccine developed by Pfizer and BioNTech.
According to Dr. June Raine, head of the MHRA, "We know from the extensive clinical trials that this was not a feature, but if we need to strengthen our advice now that we have had this experience in vulnerable populations, the groups selected as a priority, we get that advice to the field immediately."
The advisory to be vigilant was recommended after the pair of health workers "responded adversely" after being administered shots on the first day of the mass immunization rollout in the UK, NHS declared on Wednesday.
The two staff members both had with them an adrenaline auto-injector and had a history of allergic reactions. They developed symptoms of anaphylactoid reaction after being administered the coronavirus vaccine on Tuesday, reported CNN.
Powis stated the recommendation was issued on a "precautionary basis," and the individuals who had the reactions had recovered.
According to Pfizer and BioNTech, they were working with investigators to study the cases and their causes.
Dr. June Raine, head of the UK's medical regulatory agency, informed the reactions while she testified on Wednesday in front of the Parliamentary committee. The UK began inoculating medical workers and elderly people, the world's first rollout of the vaccine.
There had been two cases of anaphylaxis and one report of a probable allergic reaction since the dissemination commenced, according to the Medicines and Healthcare Products Regulatory Agency (MHRA).
On Wednesday, Canada's health regulator approved the vaccine, with Dr. Supriya Sharma, chief medical adviser at Heath Canada, touting it "a momentous occasion."
Canada is slated to receive a maximum of 249,000 doses this December. Canada officials expect to begin administering them next week as soon following their shipment from Belgium on Friday.
Related Article : New York: Who Will Be Administered the COVID-19 Vaccine First?








