A report released on Wednesday indicates potential shipments of Johnson & Johnson's COVID-19 vaccine have been suspended in the United States after a manufacturing plant mistakenly destroyed 15 million doses and posed concerns about quality control.
J&J halts COVID-19 vaccine shipment
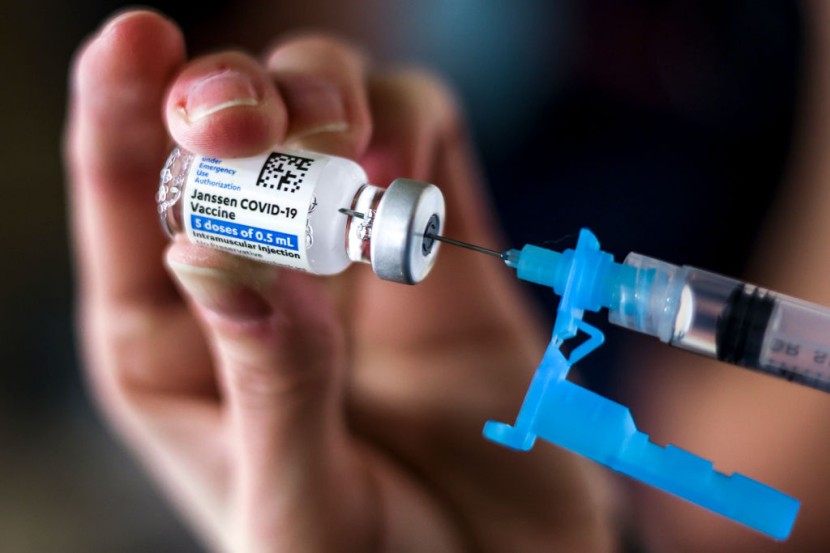
The ingredients for J&J's single-dose of COVID-19 vaccine and another inoculation were mixed up at Emergent BioSolutions' Baltimore plant several weeks ago, according to The New York Times. A report says the mistake would not impact J&J doses delivered and used around the country since they were manufactured in the Netherlands.
According to the New York Times, it has placed tens of millions of COVID-19 doses from the Baltimore plant, which are expected to be shipped over the next month in question. The Food and Drug Administration (FDA) reviews the mixup, which federal authorities have traced to human error.
Meanwhile, J&J is tightening its leverage over Emergent BioSolutions' function to prevent potential quality issues. J&J and AstraZeneca also use the biopharmaceutical firm as a manufacturing associate, NY Post reported.
White House Accuses China of Obstructing WHO into COVID-19 Origin
J&J admits failed to check a batch of COVID-19 vaccine
Johnson & Johnson said a batch of the COVID-19 vaccine failed to meet safety requirements and cannot be used. Emergent BioSolutions, one of about ten firms. J&J is using it to speed up the production of its recently approved vaccine, struggling to meet quality requirements, the company said.
The FDA has not yet approved the Emergent BioSolutions factory to produce part of the vaccine, says J&J. Emergent, a factory in Baltimore that produces bulk drug substance, has declined to comment.
J&J pledged to provide the US government 20 million doses of its COVID-19 vaccine by the end of March and another 80 million doses by the end of May. It said in a statement that it was already on track to deliver 100 million doses by the end of June and that it was aiming to deliver those doses by the end of May.
President Joe Biden has stated that he will provide enough vaccines for all adults in the US by the end of May. In addition to the 100 million shots requested from J&J, the US government has ordered enough two-dose shots from Pfizer and Moderna to vaccinate 200 million people by late May.
President Biden's target of providing enough vaccines for every adult by the end of May is still on schedule, as per the federal officials. The other two federally approved vaccines, from Pfizer and Moderna, are also being distributed as planned.
J&J hit its end-of-March deadline; its spokesman said earlier Wednesday. But he did not respond to questions regarding whether the FDA had approved the Emergent plant.
According to the US Centers for Disease Control and Prevention online vaccine tracker, J&J had given around 6.8 million doses as of Wednesday. Any additional doses could not have been reported as shipped. Still, the CDC announced on Wednesday that an additional 11 million doses of the vaccine would be available for shipment starting on Thursday.
It was uncertain where the 11 million doses came from, but Johnson & Johnson delivered completed vaccines to the United States from its factory in the Netherlands. The FDA stated that it was aware of the situation but that inquiries should be directed to Johnson & Johnson, as per KTLA 5.
Vaccine Protection Stronger Among Those Previously Infected by COVID-19, Study Reveals








