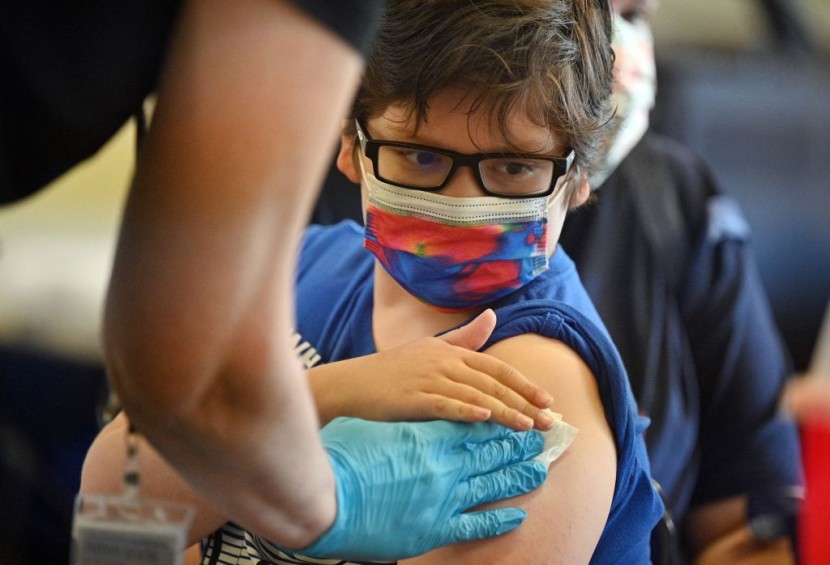
Children four years old and under could be eligible for the COVID-19 vaccine from Pfizer and BioNTech after the companies announced on Tuesday they were seeking emergency use authorization for their treatment.
The news comes as a positive thing for many parents who have been eager to have their young kids vaccinated against the coronavirus infection. However, it also raises concerns regarding personal freedom among guardians who are against vaccinations.
COVID-19 Vaccine for Children
The announcement came much sooner than the companies expected and could make more than 19 million children in the United States eligible for the jabs. The situation comes as the country faces a continued rise in pediatric coronavirus cases.
Since the beginning of the coronavirus pandemic, officials have tallied more than 10 million children in the country testing positive for the illness. Roughly 1.6 million of the cases are among kids aged younger than four.
The vaccines could offer parents a chance to return to a level of normalcy amid the Omicron surge while they are concerned about the impacts of the virus on their families. Pfizer chairman and chief executive Albert Bourla said that the pharmaceutical company believes it would likely recommend three doses to be able to provide complete protection against the virus. However, if the vaccine is authorized, a two-dose regimen can potentially ease parents' worries, as per the Washington Post.
The two companies said that they have already initiated a rolling submission of data to the U.S. Food and Drug Administration (FDA) as per the agency's request. Authorities expect that they can complete the EUA submission in the following days. They added that they would soon submit clinical data to the European Medicines Agency and other agencies worldwide.
The FDA's vaccine advisory committee is scheduled to meet on Feb. 15 to discuss the issue at hand. A member of the committee, Dr. Paul Offit, the director of the Vaccine Education Center at Children's Hospital of Philadelphia, said that the two main factors were safety and effectiveness.
Trial Results
The official added that the confidence of the American public in the vaccines depended heavily on these two factors. He said that the pharmaceutical companies should be recommending something that they would be willing to give their children. CNN reported that Offit and his team would base their decision on available data.
The situation comes as the highly transmissible Omicron variant has resulted in a record number of infections throughout the nation. It also comes after the companies received disappointing results announced in December. The data prompted officials to test a third low dose of the shot in the age group.
Government officials argued that two doses of the pharmaceutical company's vaccine were proven safe despite not providing an immune response in the whole age group. It was revealed that children involved in the trials received one-tenth of the adult dose.
Some officials reasoned that if children can already get an initial injection this month, they could be ready for a third dose once researchers achieve desired results from their tests. The first two shots of the vaccine will be given a three-week gap, while the third dose will be given two months after the second shot, the New York Times reported.
Related Article: COVID-19 Omicron Variant Warning: WHO Chief Warns Global 'Increase in Deaths,' US Death Toll on the Rise








