The United Kingdom kicked off an early winter COVID-19 and flu vaccine program on Monday, September 11, to keep up with a new flu strain and prevent another outbreak from overwhelming the country's healthcare resources.
The initiative has been expedited, according to the National Health Service (NHS) of England, because of the most recent scientific research.
Early Winter COVID-19 and Flu Vaccine Program
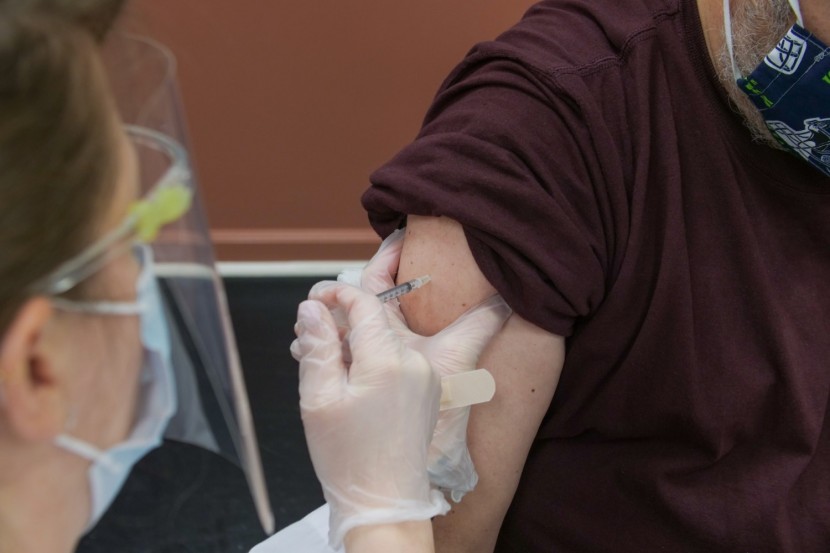
Everyone may get a flu shot. However, only certain people--such as those in nursing homes, the elderly, front-line health and social care professionals, and those at clinical risk--will be administered the COVID-19 vaccine, as reported by CNBC.
Notably, the increased hospital occupancy and record demand on staff last winter were both caused by the simultaneous spread of COVID-19 and flu.
The Royal Pharmaceutical Society voiced concern about the sudden rollout, stating that it might lead to confusion between the public and the pharmacists. Nevertheless, Scotland and Northern Ireland will begin their winter vaccination programs this month, following Wales' lead from the previous week.
After a high attack rate was attributed to Covid variation BA.2.86 (which has an unofficial Internet nickname as Pirola) in an outbreak at a care facility, the UK started investigating the strain in August.
The number of confirmed cases of BA.2.86 in England increased to 34 on September 4. Of them, 28 were residents of the care facility. None of the occurrences ended in death, although five people were hospitalized.
The study, which was published on Saturday, September 9, found no evidence that this variation had any kind of growth advantage over other variants. The UK's Health Security Agency said that there was inadequate data to determine the severity of the situation or establish a connection to precursors of increased transmission of Covid-19 in the UK.
Steve Russell, NHS director of vaccinations and screening, said, "With concerns arising over new Covid variants, it's vital we adapt the programme and bring it forward for those most at risk, and so I strongly urge everyone eligible to come forward as soon as they can for this important protection in colder months."
In the UK, four different manufacturers' Covid vaccinations are in use: Pfizer-BioNTech's, Moderna's, Sanofi/GSK's, and Novavax.
Monitoring COVID-19 Variants and Subtypes
New COVID-19 variations and subvariants, as well as the success of revised vaccinations against them, are always under the watchful eye of health agencies across the globe.
In August, the World Health Organization (WHO) warned it was keeping an eye on a strain known as EG.5, sometimes known as Eris, since it had growth advantage and immune escape properties without a verified increase in severity.
In the US, the new Covid vaccinations being distributed this autumn have been modified to better protect against the Eris strain, according to health experts.
Omicron has many subvariants, including Eris and Pirola, which contributed to a worldwide increase in cases in the latter half of 2021.








